Is it just a matter of time before TTB is scutinizing cannabis labels — for sneaky references to — wine?
I hope so. That would be great.
This article about cannabis beer got me to thinking about all manner of cannabinoid-related issues.
Did TTB approve the label yet? Not that I can see. I don’t find any Indica label approved for this Colorado brewery so far. But I do find this Dank label, which is pretty close. The label mentions a run-in with “the man” and pounds of resinous west coast Cannabacae. Dad and Dudes (the brewer) did a wonderful job of securing some trademark rights in this important term (DANK) sure to be much and more in demand in the future. But the label oddly implies that the company has a trademark registration on the term DANK in and of itself, when it does not appear that anyone does. I wonder if D&D’s registration will be sufficient, someday, to block pot purveyors from using the term DANK as part of their branding, and if pot will be considered highly-related to beer/wine/spirits.
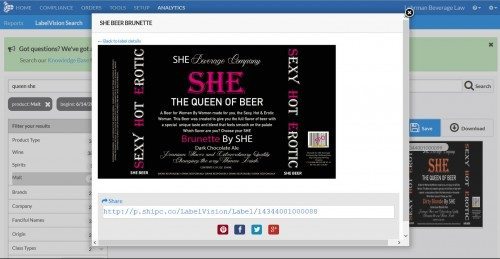
Thanks to LabelVision, it’s quite easy to see that TTB has allowed only two “cannabis” labels so far: vodka with cannabis sativa, and Cabernet Franc with notes of cannabis (“pairs...
Continue Reading Leave a Comment